Processing deadline (If there is a period for supplementation of materials, the supplementation period is not included in the processing deadline.)
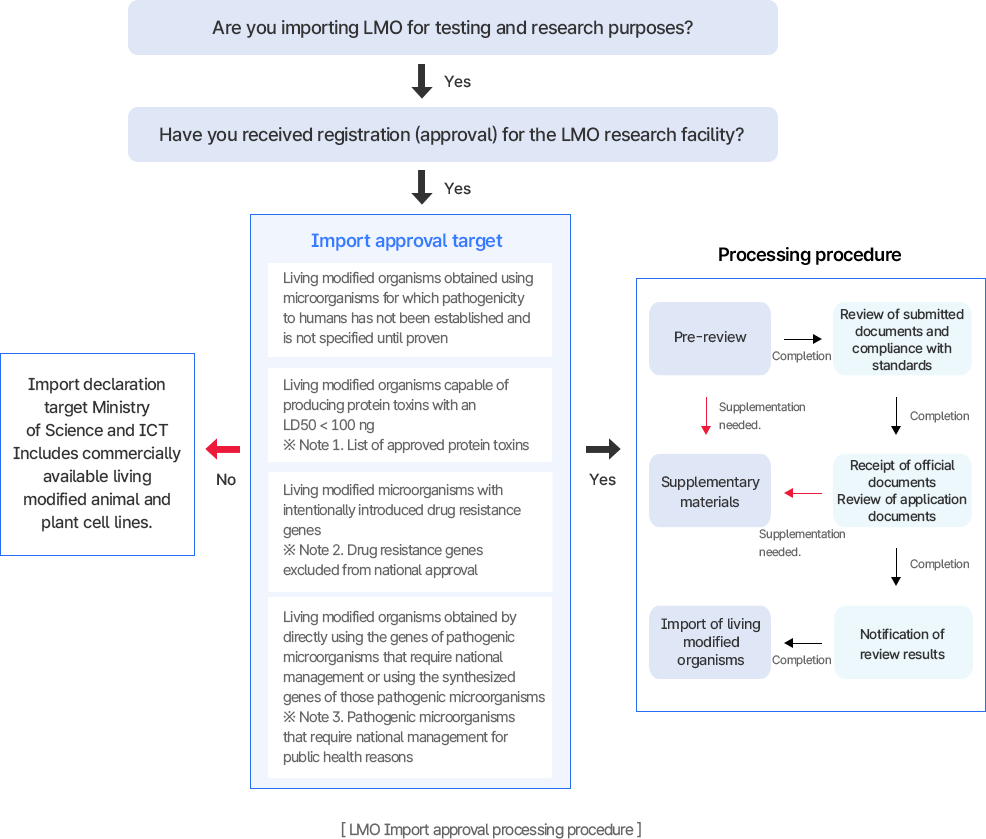
Approval for the import of living modified organisms for experimental and research purposes
For imports of living modified organisms with a high potential for harm, prior approval from the Director of the Disease Control Agency is mandatory.
- Import approval (Disease Control Agency)
- Import of LMO for experimental research that requires national management
- Import declaration (Ministry of Science and ICT)
- Import declaration (Ministry of Science and ICT)
[Management of the import of LMO for experimental and research purposes]
Categories subject to import approval for living modified organisms
- Living modified organisms obtained using microorganisms that are not specified down to the species level and whose pathogenicity to humans has not been established
- Living modified organisms capable of producing protein toxins with a lethal dose of less than 100 ng per 1 kg of body weight for vertebrates
- Integrated Announcement of the Living Modified Organisms Act [Appendix 2-1] Botulinum toxins (types A, B, C, D, E, F), tetanus toxin, shigella neurotoxin, diphtheria toxin, etc.
- Living modified microorganisms that possess intentionally introduced drug resistance genes
- Integrated Announcement of the Living Modified Organisms Act [Appendix 2-2] Drug resistance genes excluded from approval
: Living modified microorganisms developed using host-vector systems recognized for resistance to ampicillin, chloramphenicol, hygromycin, kanamycin, neomycin, puromycin, spectinomycin, streptomycin, tetracycline, or zeocin
- Integrated Announcement of the Living Modified Organisms Act [Appendix 2-2] Drug resistance genes excluded from approval
- Living modified organisms obtained by directly using the genes of pathogenic microorganisms that require national management for public health, or by using synthesized genes of those pathogenic microorganisms.
- Unified Notice for the Act on the Transboundary Movement, Etc. of Living Modified Organisms [Annex Table 2-3] [View table] Go
- Imports of commercially available living modified animal and plant cell lines must be reported to the Ministry of Science and ICT.
- Even in cases where only cultivation (propagation) is conducted using living modified organisms that have received import approval, development and experiment approval is required.
Import approval processing procedure
Documents to submit for import approval
-

- Application for import approval of living modified organisms for experimental and research purposes
- Copy of the import contract (including import agency contract) or order form
- Name, characteristics, and purpose of use of the living modified organism
- Transportation plan for living modified organisms for experimental and research purposes
- Safety management plan for living modified organisms for experimental and research purposes
- Plan for the use of living modified organisms for experimental and research purposes
- Government import stamp - Issuance for administrative fee (20,000 KRW)
- Guide to the national approval system for living modified organisms for testing and research purposes
(If there is a period for supplementation of materials, the supplementation period is not included in the processing deadline.)
- Within 60 days.
- When re-importing living modified organisms that are the same as those previously approved: Within 10 days
Changes to import approval items
- Scope of change notification and change approval
변경신고, 변경승인 표 Change notification Change approval If it falls under “minor matters” as stated in Article 8 (3) of the LMO Act, it is subject to change notification. - Change of the importer’s name, address, or contact information
Matters that do not fall under “minor matters” - Change application procedure
- Application documents: Attach the submitted documents to the change application or report for processing
- How to submit: Electronic document or email (including official documents)
- Where to submit: Biological Safety Assessment Division, Centers for Disease Control and Prevention
- Processing period(If there is a document supplementation period, the supplementation period is not included in the processing deadline.)
- Change approval: Within 60 days
- Change notification: Within 10 days
