Approval for development and experimentation using living modified organisms for testing and research purposes
To develop or experiment with living modified organisms with a high potential for harm, prior approval from the Director of the Disease Control Agency is required.
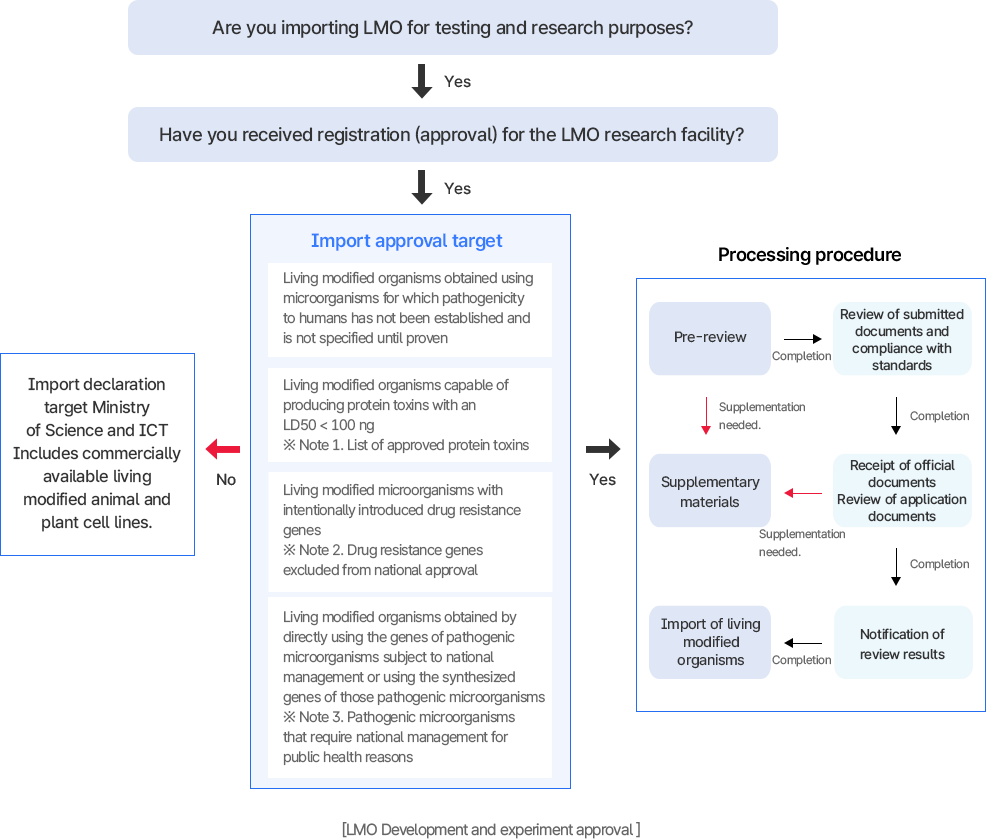
Subjects requiring approval for development and experimentation
- Where microorganisms are used that are not specified down to the species level, and for which the pathogenicity to humans has not been established
- Where genes with the ability to produce proteolytic toxins that have a lethal dose of less than 100 ng per 50% of body weight per 1 kg of vertebrates are used
- Integrated Announcement of the Living Modified Organisms Act [Appendix 2-1] Botulinum toxins (types A, B, C, D, E, F), tetanus toxin, dysenteric neurotoxin, diphtheria toxin, etc.
- Where antibiotic resistance genes are deliberately transmitted to microorganisms in a manner that does not occur naturally
- Integrated Announcement of the Living Modified Organisms Act [Appendix 2-2]Antibiotic resistance genes exempt from approval
: living modified microorganisms developed using host-vector systems that confer resistance to ampicillin, chloramphenicol, hygromycin, kanamycin, neomycin, puromycin, spectinomycin, streptomycin, tetracycline, or zeocin
- Integrated Announcement of the Living Modified Organisms Act [Appendix 2-2]Antibiotic resistance genes exempt from approval
- In cases where the genes of pathogenic microorganisms that require national management for public health are directly utilized or synthesized for use.
- Unified Notice for the Act on the Transboundary Movement, Etc. of Living Modified Organisms [Annex Table 2-3] [View table]
- Imports of commercially available living modified animal and plant cell lines must be reported to the Ministry of Science and ICT.
- Even where only cultivation (propagation) is conducted using living modified organisms that have received import approval, development and experiment approval is required.
Development and experiment approval processing procedure
이 이미지는 시험·연구용 유전자변형생물체(LMO) 개발·실험 승인 절차를 단계별로 나타낸 흐름도이다.
- 초기 확인: 개발·실험 여부 확인 → 연구시설 신고(허가) 여부 확인.
- 승인 대상:
- 병원성 여부가 불명확한 미생물을 이용한 개발·실험
- 단백성 독소 생산 능력을 가진 유전자를 이용한 개발·실험 (LD₅₀ < 100ng)
- 약제내성 유전자를 의도적으로 전달하여 개발·실험
- 국가 관리가 필요한 병원미생물을 이용한 개발·실험
- 처리 절차:
- 사전검토: 제출서류 및 기준 적합성 검토
- 자료 보완: 필요 시 보완 요구
- 공문 접수·신청 서류 심사
- 심사 결과 통보 → 유전자변형생물체 개발·실험 진행 가능
의미: LMO 개발·실험은 병원성 여부, 독소·유전자 특성 등 위험 요인을 고려해 승인 대상 여부를 판정하며, 사전검토·자료 보완 과정을 거쳐 최종 승인된다.
Documents to submit
-

- Application for approval of development and experimentation of living modified organisms
- Plan for the use of living modified organisms for testing and research purposes
- Scope of submission for risk assessment data of development and experimentation
- Supporting documents for submitted materials
- Guide to the national approval system for living modified organisms for testing and research purposes
Processing deadline
(If there is a period for supplementation of materials, the supplementation period is not included in the processing deadline.)
- Within 60 days.
Changes to development and experimentation approval details
- Scope of change notification and change approval
변경신고 및 변경승인 표 Change notification Change approval If it falls under “minor matters” as stated in Article 8 (3) of the LMO Act, it is subject to change notification. - Change in quantity of non-microbial LMO imported (volume or mass) to less than 1/100 of the original amount
- Change of importer’s name, address, or contact information
Matters that do not fall under “minor matters” - Change application procedure
- Application documents: Attach the submitted documents to the change application or report for processing
- How to submit: Electronic document or email (including official documents)
- Where to submit: Biological Safety Assessment Division, Centers for Disease Control and Prevention
- Processing period (If there is a document supplementation period, the supplementation period is not included in the processing deadline.)
- Change approval: Within 60 days
- Change notification: Within 10 days
